The Word Used to Describe Liquids That Evaporate Easily Is:
Volatility describes how easily a substance will vaporize turn into a gas or vapor. Evaporation is used to separate a solid substance that has dissolved in water or any other liquid.
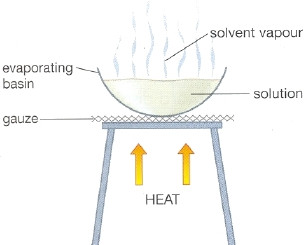
Evaporation Class 9 Matter In Our Surrounding
A volatile substance can be defined as 1 a substance that evaporates readily at normal temperatures andor 2 one that has a measurable vapor pressure.
. When heat is added vapours of both liquids move. Evaporation is the process by which water changes its state from liquid to gas or vapor. About a tenth of thick black crude oil has the complicated chemicals in it that can be changed to plastic.
The changing of liquid into vapours is called evaporation. After treatment the gases and liquids from the crude oil become hard shiny granules. All liquids evaporate except when in a closed container then no liquids evaporate.
Fractional distillation is used two separate two or more liquids that are miscible mix together easily. Use the words mass volume and density to distinguish between gases liquids and solids in terms of the particle theory of matter I dont understand what to do. The liquids that are not easily vapourizable are called nonvolatile liquids.
Learn vocabulary terms and more with flashcards games and other study tools. If we cool a gas down below its boiling point it will condense back into a liquid. The term volatile usually applies to liquids.
Learn vocabulary terms and more with flashcards games and other study tools. It is mentioned that the boxes contain the same material which is important. As the temperature increases the.
To escape as moisture or odor or gas through or as if through pores. Water boils at 212 degrees Fahrenheit or 100 degrees Celsius but begins to evaporate at 32 degrees Fahrenheit or 0 degrees Celsius only slowly. Some examples of liquids that can evaporate at room temperature are alcohol gasoline and water.
If a saturated hot solution is. Immiscible refers to liquids that will not dissolve in each other. Water does not behave as other materials as the solid phase is actually less.
It is one of the fundamental procedures each chemist must master to become proficient in the laboratory. Like petrol how easily a liquid evaporates is referred to as its volatility. Evaporation is the process when molecules from liquid pass to the atmosphere as gas without reaching the boiling point.
Other students are comfortable with seeing powder as a solid because it will not wet immersed objects. The other liquids are usually organic solvents. In conduction increased kinetic energy is passed from molecule to molecule.
Am I supposed to describe the 62995 results Science. Miscible refers to two liquids that will mix together in any amount. But we use the word to mean a group of materials that are manufactured from oil.
The intermolecular forces between molecules are relatively weak in liquids that are volatile so the particles dont need to much kinetic energy to escape from the surface of your skin. Convection is the transfer of heat by a large-scale displacement of groups of molecules with relatively higher kinetic energy. Start studying chemistry ch12 liquids solids and intermolecular forces.
Separation by Evaporation. In convection molecules with higher kinetic energy are moved from one place to another place. A scientist is working in a lab and accidentally combines two liquids that quickly form a solution.
You could use the word transpirable. Water can make other liquids wet. The dissolved substance is left as a solid residue when.
What is a solvent called if it evaporate easily at room temperature. This happens at its boiling point. Chemists apply the term to water in an unusual way.
What is separation by evaporation. Substances which appear as powders or in fine granules like sand or talc are often identified as liquids because they are viewed by students as easily shaped or freely poured. To emit or give off waste matter watery vapor etc through the surface as of the body or of leaves.
Start studying Chemistry Flashcards. Plastic can be coloured melted shaped squashed stretched rolled into sheets or pulled into. Alternatively you could use respirable regarding ghosts or mists as defined by the Merriam-Webster.
Nonvolatile liquids that do not evaporate easily Motor oil is virtually from CHEMISTRY MISC at Lee University. A liquid with a high vapor pressure can evaporate. A liquid can evaporate at any temperature.
Recrystallization is based on the principles of solubility. Those liquids that evaporates at normal temperatures are called volatile liquids. Water and water based liquids for example milk sea water cordial and lemonade are.
The most volatile materials are those that most easily evaporate eg. Water is specifically not mentioned in this activity as it is an exception which will be discussed later. Recrystallization is a technique that chemists use to purify solid compounds.
Water H2o is a. A wet solvent is usually a bad thing and the term is used even if the amount of water is very small usually it is employed when the. The rate of evaporation depends on the room temperature and a property of liquids called vapor pressure.
This activity is used to explain the general property that solids are more dense than liquids which are more dense than gases. Fractional distillation uses beads in the fractionating column gives a larges surface area for hot vapour to condense. Compounds solutes tend to be more soluble in hot liquids solvents than they are in cold liquids.
However if we heat it enough it will eventaully boil and turn into a gas. Which process could be used to separate the two liquids1 point. One of the reasons for these solvents is that the presence of water is a bad thing for whatever the chemist is trying to do.
We use the word condense to describe what happens when a vapour or a gas turn back into a liquid. The mixed liquids have narrow but different boiling points.

Evaporation Chemistry For Non Majors

What Is Evaporation Definition Facts And Examples Twinkl
Why Do Liquids Evaporate When They Aren T At Their Boiling Temperature Quora
Comments
Post a Comment